Variations of specific heat (plot a) and thermal conductivity (plot b)... | Download Scientific Diagram
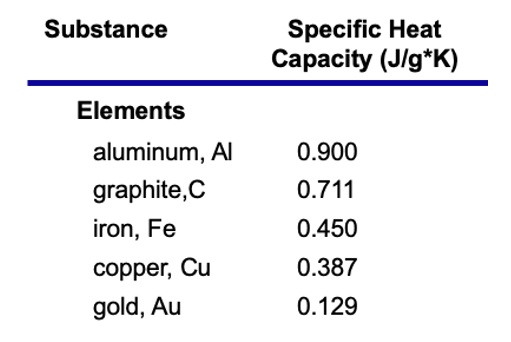
SOLVED: Substance Specific Heat Capacity (Jlg*K) Elements aluminum, Al graphite,C iron, Fe copper; Cu gold, Au 0.900 0.711 0.450 0.387 0.129

Comparison between the calculated and experimental heat capacity of... | Download Scientific Diagram
A 36.07 g sample of a substance is initially at 27.8°C. After absorbing 2639 J of heat, the temperature of the substance is 109.0°C. What is the specific heat of the substance? | Socratic
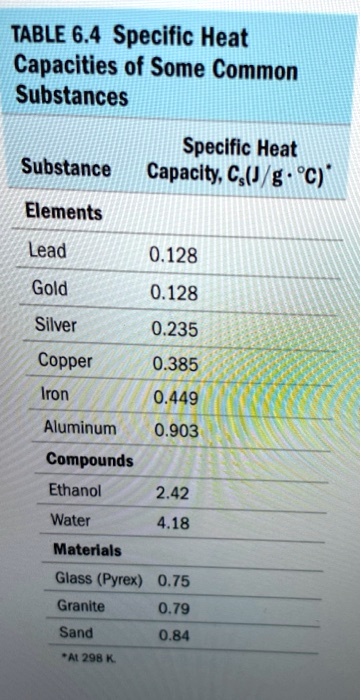
SOLVED: TABLE 6.4 Specific Heat Capacities of Some Common Substances Specific Heat Substance Capacity; C,(J / g . *C) Elements Lead 0.128 Gold 0.128 Silver 235 Copper 0.385 Iron 0.449 Aluminum 0.903
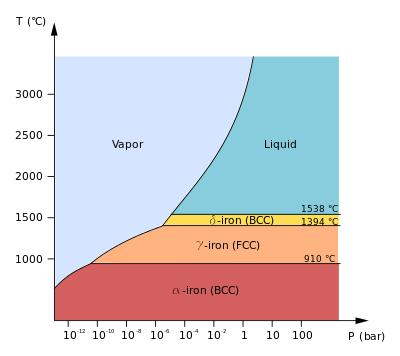
Molten iron is extremely hot, averaging about 1,500 C. The specific heat of iron is 0.46 J/gC. How much heat is released to the atmosphere when 1 kg molten iron cools to
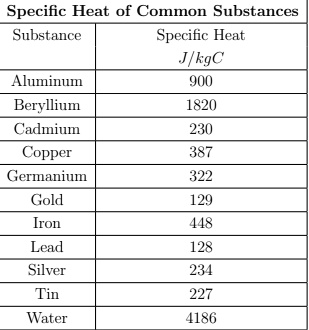
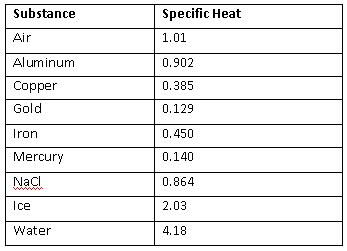
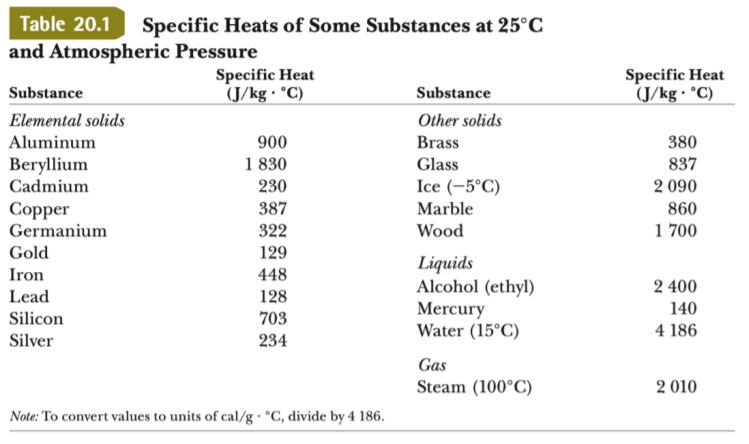
SOLVED: Specific Heat of Common Substances Substance Specific Heat (J/kg°C) Aluminum 900 Beryllium 1820 Cadmium 230 Copper 387 Germanium 322 Gold 129 Iron 450 Lead 128 Silver 235 Tin 227 Water 4186

Derived mean values of the specific heat of pure iron in comparison... | Download Scientific Diagram
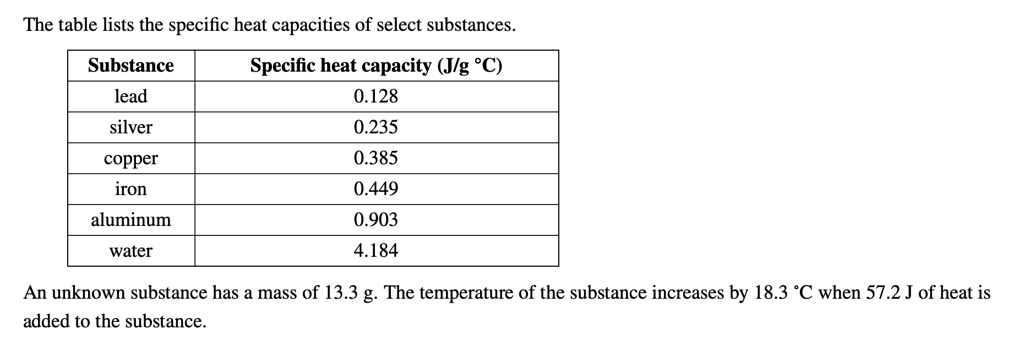
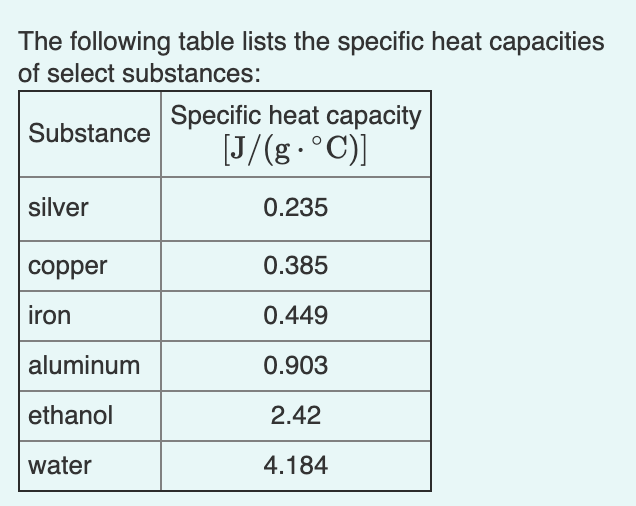
SOLVED: The` table lists the specific heat capacities of select substances Substance Specific heat capacity (Jlg C) lead 0.128 silver 0.235 copper 0.385 iron 0.449 aluminum 0.903 water 4.184 An unknown substance
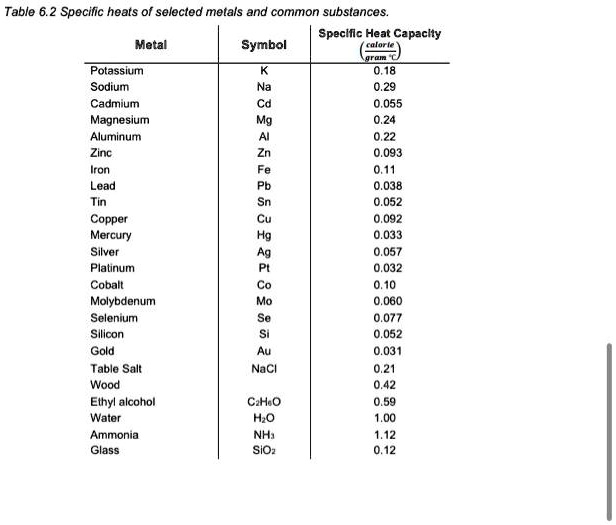
SOLVED: Table 6.2: Specific heats of selected metals and common substances Metal Symbol Specific Heat Capacity Potassium K 0.18 Sodium Na 0.55 Cadmium Cd 0.93 Magnesium Mg 0.11 Aluminum Al 0.038 Zinc





![ANSWERED] Metal Specific Heat (J/g°C) Calcium 0.647 ... - Organic Chemistry ANSWERED] Metal Specific Heat (J/g°C) Calcium 0.647 ... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/75254282-1659635207.98119.jpeg)