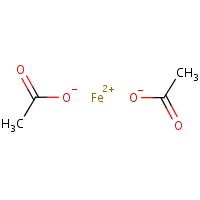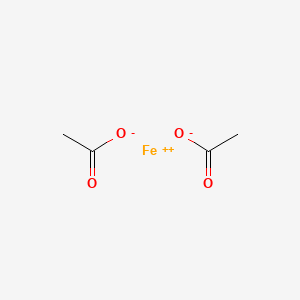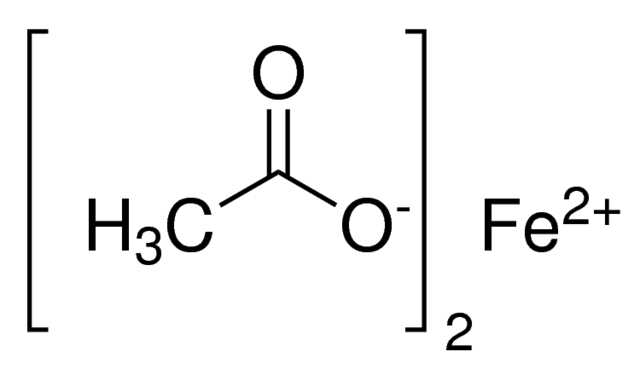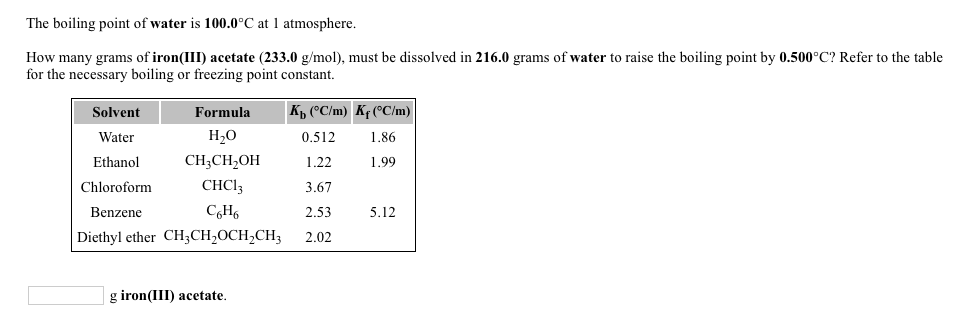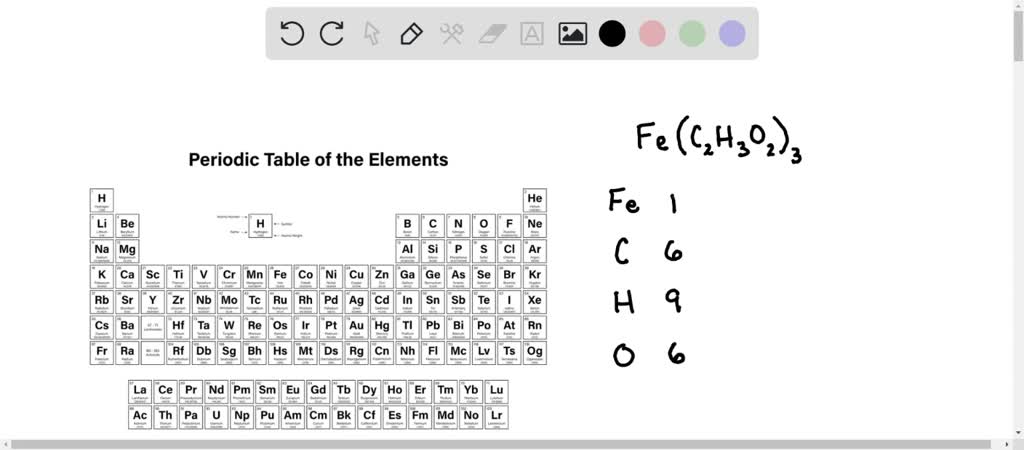
SOLVED: What is the molar mass of iron (III) acetate? (Please show any work to arrive at an answer. )
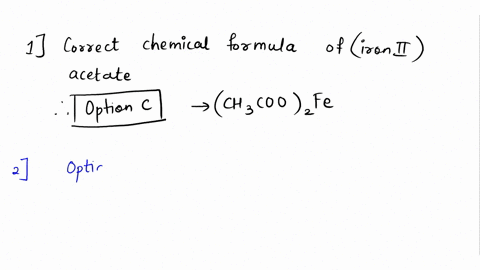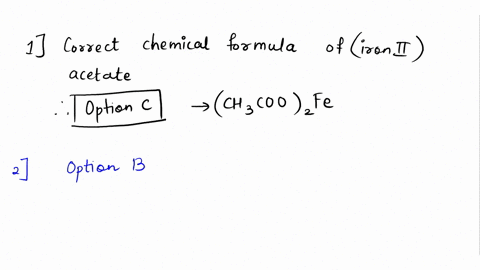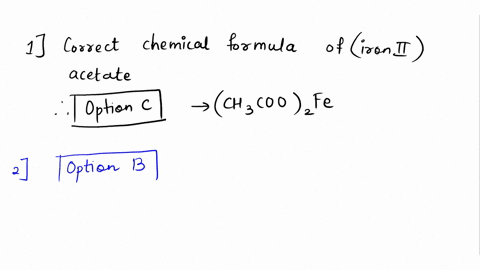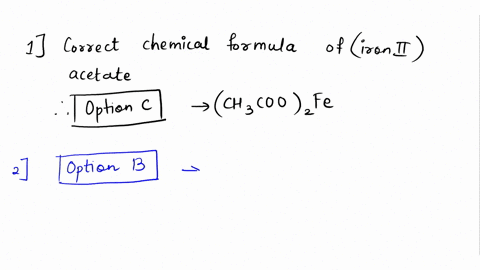
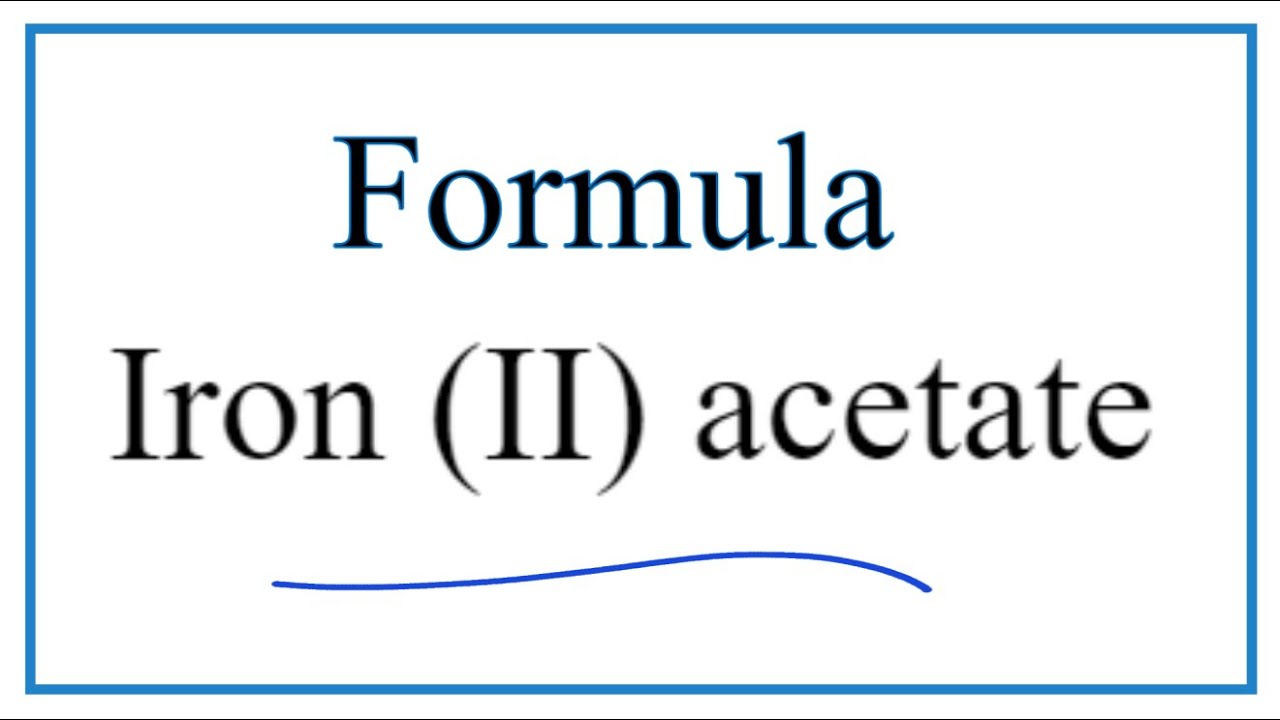
SOLVED: Please answer b and c Compare the color of iron(II) acetate to that of iron(III) acetate. (a) What color change did you observe? - ANSWER is the color went from colorless

Single crystal growth, structural characterization and magnetic properties study of an antiferromagnetic trinuclear iron(III) acetate complex with uncoordinated hexamine - ScienceDirect
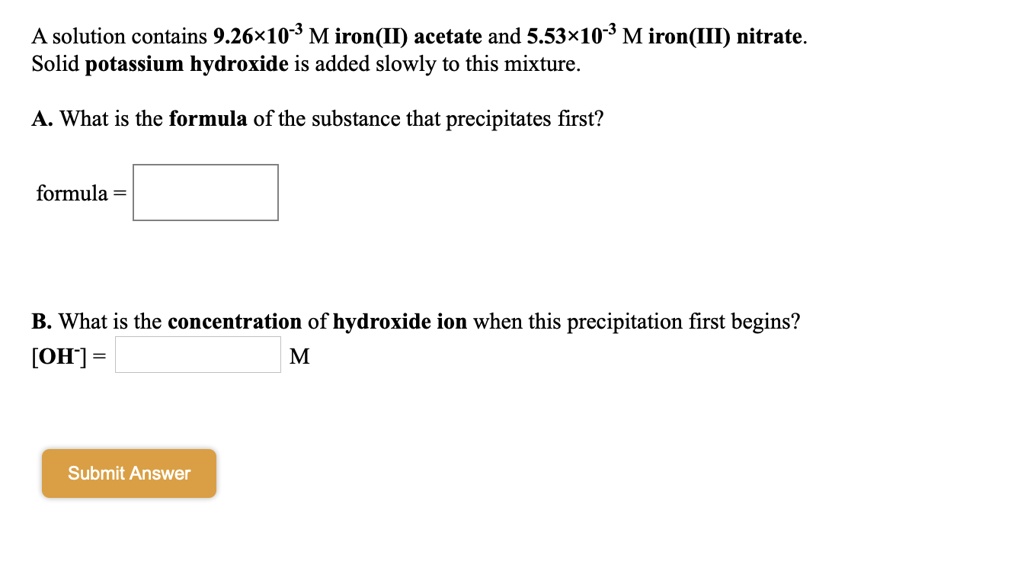
SOLVED: A solution contains 9.26*10-* M iron(II) acetate and 5.53x10-* M iron(III) nitrate. Solid potassium hydroxide is added slowly to this mixture What is the formula of the substance that precipitates first?

Compounds vs. Elements Compound Table Salt : Soluble crystals, stable, edible Elements (Components) Sodium – shiny metal, reactive, poisonous Chlorine. - ppt download